Carbon Capture, as the name suggests, is the domain of technology that focuses on capturing carbon directly out of air, in the form of the carbon dioxide (CO2) and methane.
Carbon capture technologies offer us a solution at a critical point in our history to mitigate the environmental impact of CO2 emissions, particularly from energy production facilities, such as power plants and industrial facilities. With CO2 emissions rising, and global warming gaining recognition as being linked to the higher concentrations of the gas, carbon capture technologies have been (rightfully) gaining more significance - while becoming important drivers of mitigating climate change and maintaining a healthier planet.
One of the primary sources of CO2 emissions is the combustion of fossil fuels for energy generation. To address this challenge in the past, companies and governments put a lot of focus into pre-combustion and post-combustion facilities. There are certain benefits to both technologies - we’ll cover these before moving onto why they’re ultimately problematic.
Pre-combustion
Pre-combustion is fascinating. But we can almost guarantee you’ve not come across it much, if at all. So let’s start there!
As the name suggests, pre-combustion capture involves removing carbon from the fuel source itself before combustion of the fuel occurs. The first step of this is to take a solid or liquid fuel, like coal, biomass, or heavy hydrocarbons, is converted into a gas mixture - known as syngas (from synthetic gas). This gasification process is usually done using a controlled amount of oxygen, and/or steam.
This process converts the fuel into CO₂, CO and H₂ - with only the latter of which being “clean”. The carbon monoxide in particular is the most harmful of the lot, and so has to be converted to CO₂ first. This is done via a shift reaction with yet more steam, whereby the CO reacts with H₂O to form CO₂ and H₂. The syngas mix now contains just CO₂ and H₂, meaning if you can remove the CO₂, you now have essentially just a clean fuel. This is exactly what happens, using an (aptly-named) carbon dioxide absorber.
This sounds convoluted, perhaps; but the gist of it is that carbon-rich fuels have CO₂ siphoned from them in advance, leaving behind a hydrogen-rich gas that can be burned more cleanly. So to recap;
• You begin with a carbon-based fuel (e.g., liquid alkanes like methane or other hydrocarbons).
• The fuel is converted into syngas (CO and H₂).
• CO in the syngas is converted to CO₂ and more H₂.
• CO₂ is captured, leaving primarily hydrogen.
• Hydrogen is used as a fuel, which, when burned, produces only water vapor as a byproduct.

This approach can potentially capture a higher percentage of CO2 compared to post-combustion methods, and yields a more concentrated CO2 stream. This in turn allows for easier separation.
The problem with this, of course, is that the fuel is effectively converted to H₂, and thus is only compatible with turbines, engines and generators that are designed specifically to use H₂.
Yet, CO₂ emissions produced this way could instead be handled and processed in a centralised location, rather than being created at the point of combustion. Imagine all of the pollution caused by cars and jet planes instead being concentrated in a single facility, which could then be contained and converted without ever being exposed to the atmosphere. Much more manageable!
The problem is how you then choose to use it. More on that in a moment. Next, let’s cover post-combustion.
Post-combustion
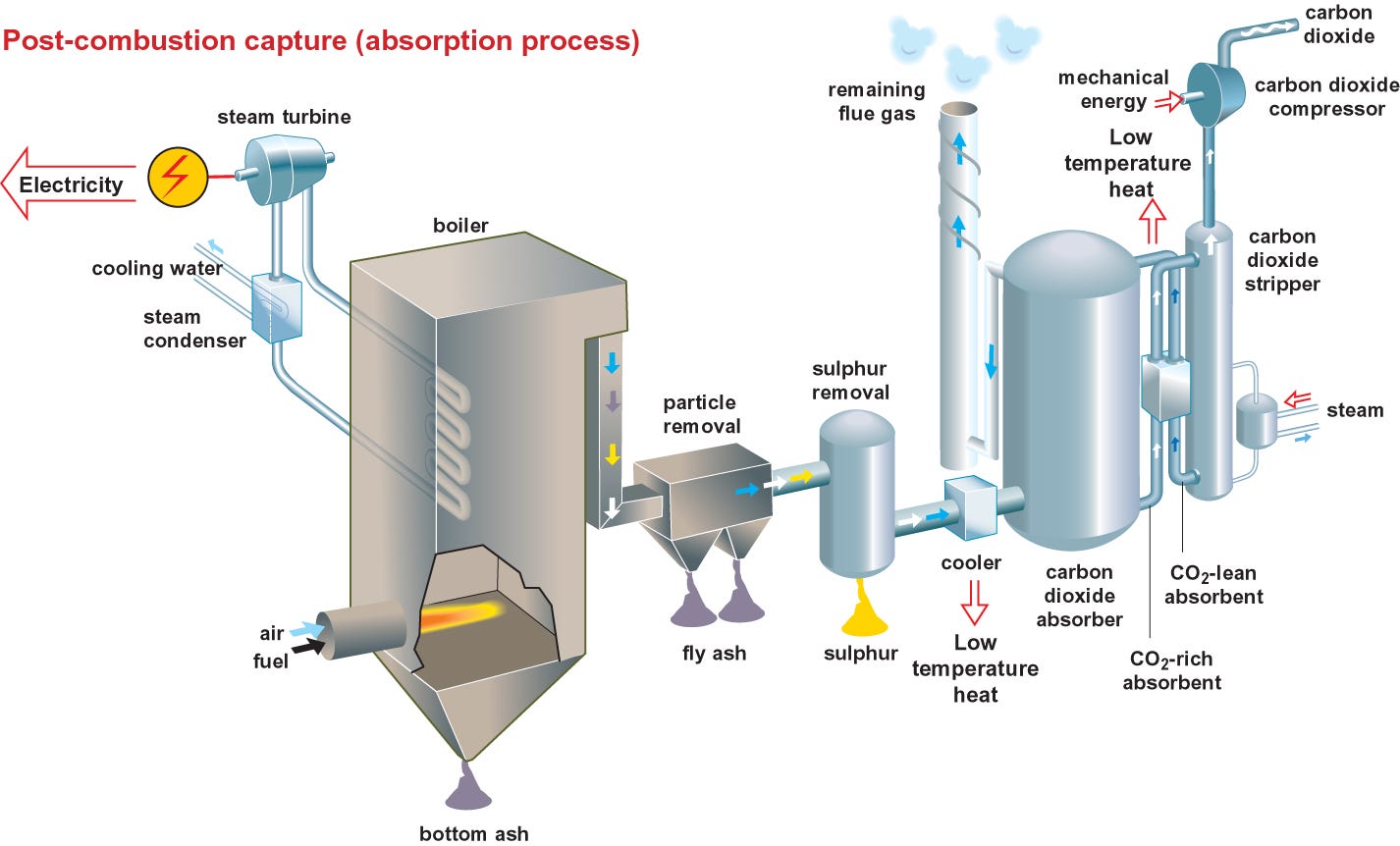
This one you probably have heard of, though not necessarily in the way we’re about to cover. Rather than looking at modifying trees or microalgae to more efficiently convert CO₂ to biomass (as we looked at in our previous articles), post-combustion CO₂ removal is a process used to capture CO₂ from the exhaust gases (flue gases) produced after burning fossil fuels, such as those released from power plants and industrial facilities. The ostensible goal being to reduce the amount of CO₂ emitted into the atmosphere.
The technical side of this is also quite interesting, so let’s cover that quickly too.
It starts with the aforementioned flue gases. These are then passed through some kind of medium capable of absorbing CO₂. There are a few ways of doing this that see the most use - chemical solvents such as amines, solid absorbents such as activated carbon, and selective membranes that allow CO₂ to pass but block other flue gases. There’s even cryogenic distillation, whereby the flue gases are cooled to very low temperatures to separate CO₂ via condensation.
The remaining flue gases are then depleted of CO₂, meaning they can continue moving on for further processing or release into the atmosphere.
So what’s the problem? This seems relatively benign, right? Good for the planet, even! Unfortunately, no.
The problem lies at the core of how CO₂ is being treated here. Not chemically, but as an overall concept. It treats CO₂ as waste. Once distilled, it now has to be dealt with. A neutral form of this is through storage facilities, which effectively store the CO₂ beneath the ground. But there are much more sinister uses.
Frequently, CO₂ is injected deep into underground rock formations. This is often prone to leakage - contaminating ground water with carbonic acid. And even when it doesn’t leak, the process scales poorly: ensuring that the CO₂ remains securely stored requires long-term monitoring and maintenance. Not only this, but there are only so many geological sites that are even suited to storing CO₂ in the first place, and we would quickly run out. Worst of all, it can even potentially lead to seismic activity - i.e., earthquakes.
Worse yet is Enhanced Oil Recovery. This is where CO₂ is used to extract additional oil from otherwise depleted oil fields. This literally increases overall fossil fuel production, thereby going against the goal of reducing reliance on fossil fuels. But as a carbon capture facility is in the mix, this still gives the misleading impression of it being green - hence is just another example of greenwashing. We covered greenwashing in a previous article, and unfortunately even in the modern age of instant information, this phenomenon is only becoming more and more prevalent.
If you’re willing to put up with some crude language, our friends at JuiceMedia have put together a video that pretty much sums it up (we recommend skipping to 3:18 for the section on enhanced oil recovery):
What’s the solution, then? Well, to us at ValleyDAO, it’s simple. We need to stop seeing CO₂ as a waste product. We can do this by getting back into harmony with the carbon cycle. We covered this in an article, too.
References:
https://www.mdpi.com/2077-0375/13/12/898
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8348380/












